What has Madrid got in its pocket for health autonomy?
As anticipated, Madrid is racing to finalise its ‘Open Strategic Autonomy’ (OSA) non-paper by October. In this edition of Acumen Trends, we review what we have learned so far about the content of the draft for the health sector, as well as the initial comments from other Member States.
Key Takeaways
- The Spanish Presidency’s OSA initiative, based on five ‘Is’ (internal production; integration; innovation; internationalisation; internal confidence), will comprise three pillars and recommended actions in strategic sectors, including health.
- With regard to health, the Presidency is considering four sector-specific measures: (i) reshoring of production of active pharmaceutical ingredients (APIs); (ii) creation of common strategic stockpiles; (iii) development of emergency production capacities; and (iv) introduction of compulsory cross-border solidarity.
- The initiative will be discussed by EU leaders at the next informal European Council on 6 October. While it remains unclear whether the recent snap elections will diminish the Presidency’s political clout on important issues such as OSA, the upcoming Belgian Presidency is certainly worth watching.
In details
1. A broad-spectrum Spanish plan
According to Madrid, the concept of OSA is accepted as an imperfect but useful reference by all Member States and can be defined as the capacity to act autonomously when and where necessary, and with partners in areas where this is possible. Based on this approach, the Spanish non-paper, developed in close consultation with Member States and the Commission, focuses on five priorities of equal importance:
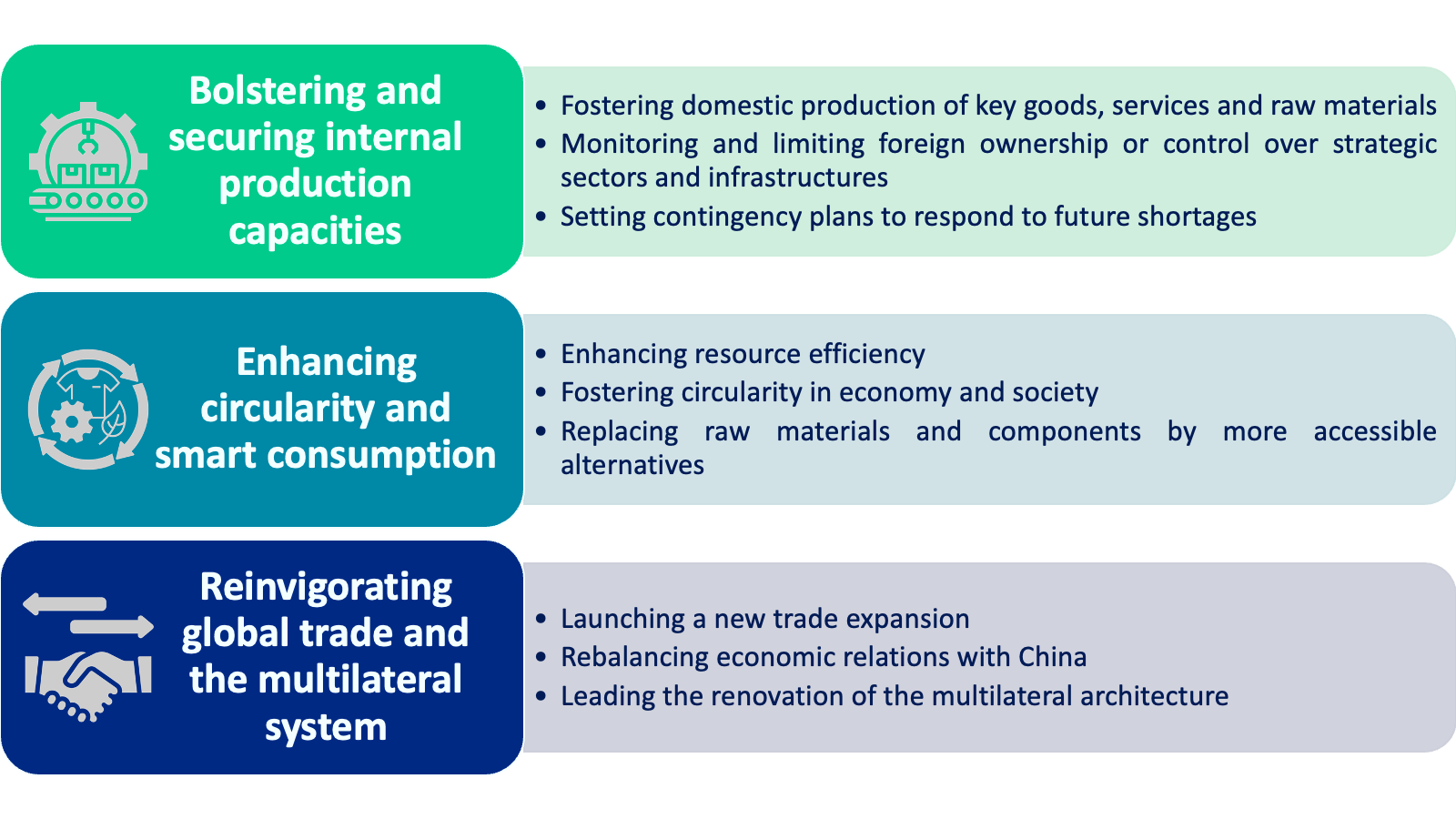
- Internal production – EU-based production should be promoted, including through public-private partnerships. This industrial effort will also need to be targeted and adapted to high value-added goods and services where the EU (i) already has a competitive advantage, (ii) has the potential to become a world leader, or (iii) needs a minimum level of capacity to ensure its future economic security.
- Integration – To ensure its economic security and global leadership, the EU will need to make concrete and substantial progress towards greater integration, including the completion of a resilient, strengthened and fully operational internal market. While national interests and sovereignty must be respected, coordination will be paramount so as not to undermine Europe’s unity and strength.
- Innovation – To ensure that currently imported goods, services, and raw materials are replaced by competitive and sustainable alternatives, the EU will need to make a strong commitment to R&D and the adoption of new technologies and organisational forms, while respecting the precautionary principle. It will also be essential to support capacity building in areas such as new infrastructure and skills.
- Internationalisation – The EU should pursue a three-pronged strategy:
- First, to promote the improvement of the multilateral system and the strengthening of mechanisms for dealing with non-compliance, resolving disputes, and enforcing global rules.
- Second, to launch a new trade expansion aimed at rebalancing, diversifying, reorganising, and expanding its economic relations, primarily with like-minded partners, but also with low- and middle-income countries.
- Third, to strengthen its capacity to act swiftly and decisively as a geopolitical entity, by shaping the economic environment of external actors that threaten its security.
- Inner confidence – Madrid sees the EU’s future as leading a new era of global prosperity through its cutting-edge technology companies, clean and affordable energy, high quality healthcare, affordable and nutritious food, higher incomes, and better living standards.
How can we expect these priorities to be delivered? Building on the agreements reached by EU leaders in Versailles in March 2022, Madrid will present a comprehensive, balanced and forward-looking strategy to ensure the EU’s economic security and global leadership by 2030 in four critical sectors (energy, digital technology, health and agriculture) around nine action lines:
2. Health specific zoom
Health, one of Europe’s key strategic sectors, is given particular attention in the Spanish non-paper, with four main areas of action.
Firstly, reshoring of APIs production. While it is not in Europe’s interest to produce locally everything it currently imports, Madrid deems it vital for the EU to have adequate production capacity for APIs as they are crucial to its future economic security. The production of innovative medicines should also be reshored, as the EU already has a competitive advantage in this area (ongoing French reshoring efforts should be monitored in this respect). The European Parliament study on possible measures to facilitate the production of APIs should be an important reference for Spain.
Secondly, the creation of common strategic stockpiles. Spain is calling for the extension of the rescEU to include critical medical countermeasures – including pharmaceuticals – as we envisaged in our in our previous issue (see Putting Europe back on the path to ‘Open Strategic Autonomy’). As a reminder, the EMA is currently working on a list of critical medicines based on two risk criteria: (i) the severity of the therapeutic indication and (ii) the availability of alternatives. Work has already started to apply this methodology to products published in national lists of critical medicines (e.g., in France, Portugal, Croatia and the Netherlands) and in the EMA’s medicines databases, and a first version will be published by the end of 2023.
Thirdly, the development of emergency production capacities. In Spain’s view, the EU should encourage Member States to develop joint and national emergency production capacities, to be established on a permanent basis with public support from national or European institutions (e.g., tax exemptions or public procurement). The EU FAB network, recently established by DG HERA, is a clear model for such a system. Another solution proposed in the Spanish non-paper seems to be based on the concept of evolutive smart factories that can be reconfigured in less than 15 days to produce new medicines and vaccines.
Fourthly, the introduction of compulsory cross-border solidarity. Recalling the restrictions on intra-EU exports during COVI-19, the Spanish Presidency would introduce new rules to ensure that solidarity between Member States is compulsory in certain situations (e.g., health emergencies). Madrid would also clearly prohibit unjustified restrictions on intra-EU exports. It should be noted that the Belgian call for a Critical Medicines Act refers to a voluntary solidarity mechanism that is already being implemented under the supervision of the EMA: Emer Cooke, the Agency’s Executive Director, reported at the recent informal EPSCO Council that 11 countries had volunteered to form a dedicated Working Group.
3. EPSCO’s informal views
While we have covered the full content of the informal discussion of EU health ministers in a previous alert (see 28 July informal EPSCO meeting readout), here we focus on national comments on possible actions at EU level that the Spanish Presidency might refer to in its non-paper.
Starting with the Critical Medicines Act, 11 Member States (AT, BE, CY, CZ, DE, FR, HU, IE, NL, PL, RO) explicitly supported it and some went into more detail:
- Germany proposed new public procurement rules to encourage investment in the EU.
- France stressed the need for a more level playing field in environmental regulation.
- Hungary called for greater harmonisation of the various pieces of legislation affecting pharmaceuticals, such as the Net Zero Industry Act and the F-Gas Regulation.
With regard to the ongoing revision of the European pharmaceutical legislation, several ministers (CY, DK, FI, IE, LI, SE, SK) recalled its important and expected impact on Europe’s security of supply. Although Austria did not intervene, it is worth recalling its previous comment during the informal COMPET meeting: for Vienna, the revision takes the wrong approach by focusing only on public health, but not on its impact on the pharmaceutical industry and global competition.
Finally, given the complexity of pharmaceutical supply chains, three Member States (CZ, IE, MT) stressed the “open” aspect of OSA and recalled that production should not be limited to the EU, but rather diversified. However, Malta and Estonia also stressed the need for profitability for older and off-label medicines, which is likely to have an impact on prices.
4. The way forward
As José Manuel Miñones, Spain’s acting health minister, confirmed on 28 July, Madrid’s non-paper on OSA will be considered by EU leaders at the next informal European Council on 6 October. Priorities for the EU’s next Strategic Agenda for 2024-2029 will also be discussed, focusing on competitiveness, the Single Market and OSA.
Meanwhile, Spanish MEPs Dolors Montserrat (EPP), Nicolás González (S&D) and Susana Solís (Renew) are collecting signatures in support of their letter to the European Commission calling for a Critical Medicines Act. If they manage to collect 36 signatures by 1 September, an oral question on the subject could be put on the agenda of the September plenary.
Depending on how the document is received, a more in-depth debate could take place at a formal European Council before the end of 2023, either on 26-27 October or 14-15 December.
It remains to be seen whether Spain will have the means to fulfil its ambitions as recent snap elections have raised doubts over what Spain can achieve during its mandate: while political meetings, working groups and sectoral conferences will go ahead as planned, the Presidency risks losing some political weight on important files, including OSA.
The Belgian Presidency in the first half of 2024 will really be worth watching: With resilience as its overarching theme, and a public health conference on the subject on 26-27 March, Brussels may be tempted to go further and work on Council conclusions or even a Council resolution (see Putting Europe back on the path to ‘Open Strategic Autonomy’).